|
| Case Series | ||||||
| Two cases of renal cirsoid arteriovenous malformations combined with aneurysmal types: Angiographic findings and endovascular treatment | ||||||
| Toh Yamamoto1, Zenjiro Sekikawa2, Izumi Torimoto3, Shigeo Takebayashi4 | ||||||
| 1Department of Diagnostic Radiology (Attending Physician), Yokohama City University Medical Center, Japan 2Department of Diagnostic Radiology (Assistant Professor), Yokohama City University Medical Center, Japan 3Department of Diagnostic Radiology (Research Fellow), Yokohama City University Medical Center, Japan 4Department of Diagnostic Radiology (Professor), Yokohama City University Medical Center, Japan | ||||||
| ||||||
| [HTML Abstract]
[PDF Full Text][Print This Article] [Similar articles in PubMed][Similar articles in Google Scholar] |
| How to cite this article |
| Yamamoto T, Sekikawa Z, Torimoto I, Takebayashi S. Two cases of renal cirsoid arteriovenous malformations combined with aneurysmal types: Angiographic findings and endovascular treatment. Edorium J Radiol 2018;4:100011R02TY2018. |
| ABSTRACT |
|
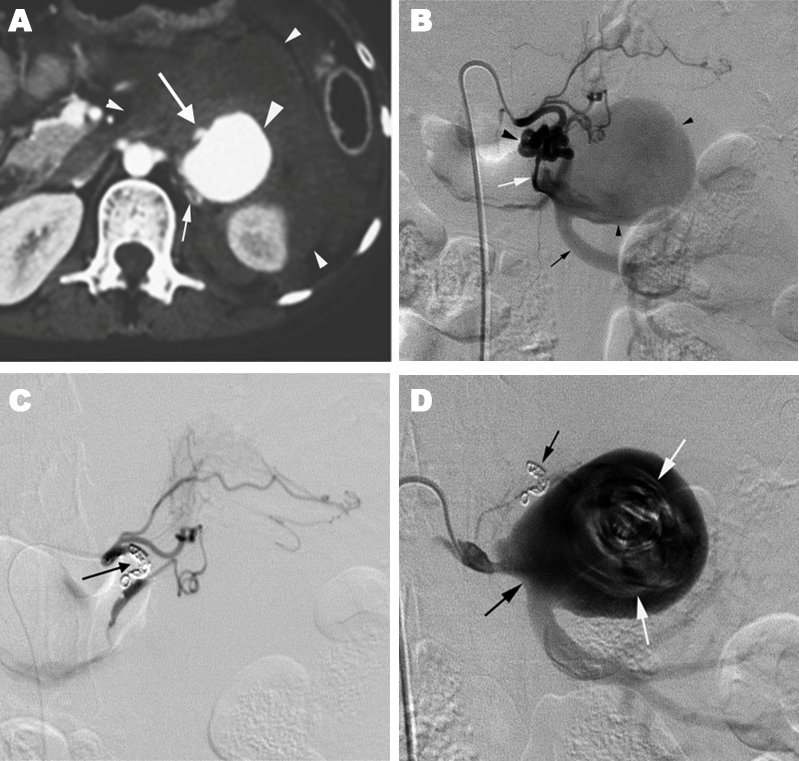
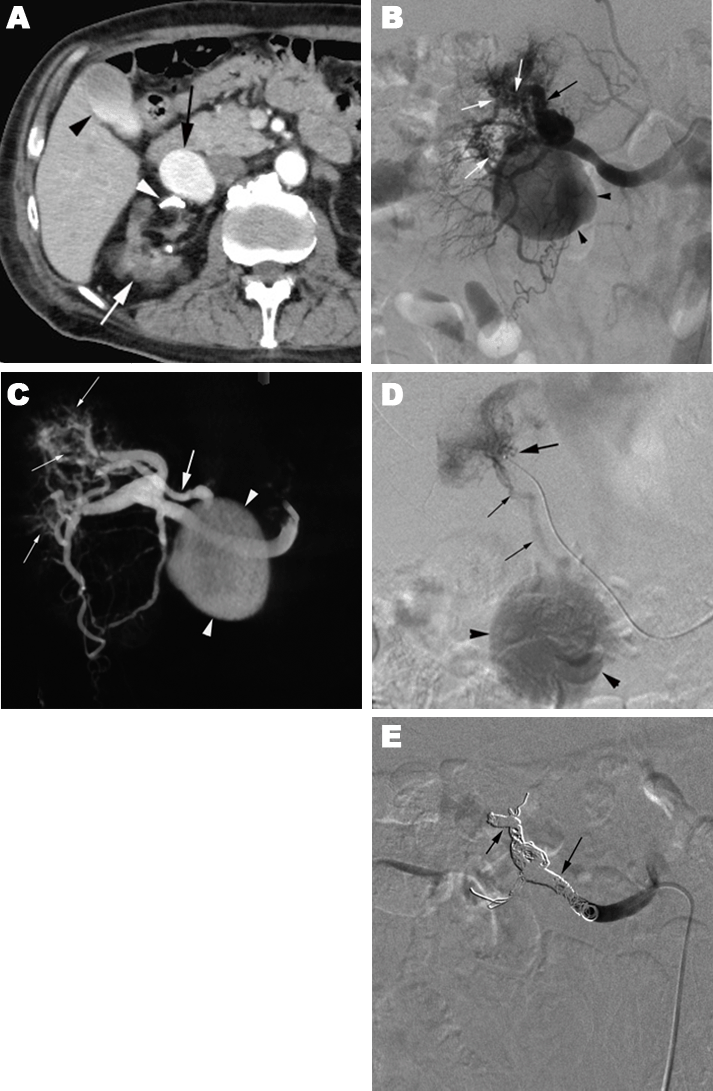
Introduction: We report two cases with a new subtype renal arteriovenous malformation (AVM) and describe their angioarchitecture and endovascular therapy. Case Series: In a 31-year-old postpartum patient with a sudden onset of hemorrhagic shock, CT showed a large renal arterial aneurysm and a retroperitoneal hematoma. Pre-embolization angiography showed that the left middle adrenal artery had a cirsoid AVM with a fistula to a large saccular aneurysm arising from the left main renal artery. Her vital sign was stable after a complete occlusion of the cirsoid arteries by the deployments of microcoils. But coil packing of the aneurysm was limited to partial because too many detachable coils were needed. Finally, a partial aneurysmectomy of the residual lesion was performed. A 79-year-old man who had undergone chronic hemodialysis had a right renal aneurysm, 4 cm in diameter, which was diagnosed as a venous aneurysm in a dilated draining vein of cirsoid type AVM Coil embolization of the main renal artery and the segmental artery feeding the AVM was performed resulting no visualization of the aneurysm on angiography. However, follow-up CT showed residual flow in the aneurysm. Conclusion: Both diagnostic and interventional radiologists should pay an attention to a new subtype of renal cirsoid AVM which is combined with aneurysm. Keywords: Aneurysm, Arteriovenous malformation, Embolization, Kidney |
| INTRODUCTION |
|
Arteriovenous malformation (AVM) is a vascular disorder which has communications between arteries and veins with a vascular nidus that bypass the capillary bed. Renal AVMs are rare and usually divided into cirsoid, and aneurysmal types although some authors classified the AVMs into three types including angiomatous type [1]. The cirsoid AVM, which has usually multiple feeding arteries with mild dilatations and dilated draining veins, was reported to be less than 0.04% of incidence rate in the general population [2]. Aneurysmal type AVMs, which are further rare, are composed of a single feeding artery and a single draining vein with aneurysmal dilatation. The lesion may mimic renal arterial aneurysm because both diseases are discovered incidentally during imaging such as computed tomography (CT), magnetic resonance imaging, and ultrasonography [3]. In this article, we report two cases with a new subtype renal arteriovenous malformation (AVM) and describe their angioarchitecture and endovascular therapy. |
| CASE SERIES |
|
Case 1 Case 2 |
|
|
|
|
DISCUSSION |
|
The cirsoid AVM causes massive hematuria due to increased peripheral venous pressure resulting rupture venous-calyx communication [4], [5]. It has been thought to be congenital in etiology. However, there is a diagnostic dilemma that the differentiation of congenital lesion from acquired lesion is difficult because a long-term AV fistula by trauma or inflammation can be associated with cirsoid vessels. While the aneurysmal type AVM causes no symptom other than congestive high flow heart failure which may sometime occurs. The latter type malformation is believed to be associated with a congenital arterial or venous aneurysm that may expand and eventually eroded into a vein or artery and result in an AV fistula [6]. We have experienced three subtypes of the aneurysmal type AV M: Type 1, arterial aneurysm with an AV shunt; Type 2, arterial aneurysm with an AV shunt to the vein with distal stenosis; Type 3, arterial aneurysm with an AV shunt to venous aneurysm. Case 1 had a cirsoid AVM which was connected to a renal arterial aneurysm. We could not determine whether the connection was acquired or congenital. Case 2 had the venous aneurysm in the draining vein of the cirsoid AVM. Suggestively, the aneurysm was caused by a stenosis in the distal portion of the draining vein of the AVM. Advances in endovascular therapy have allowed interventional radiologists to contribute to the management of the two typed renal AVMs. Endovascular techniques now provide an alternative method for treatment, with low morbidity and recurrence rates [7], although improvement of surgical techniques and materials allows renal main trunk arterial aneurysms repairs [8]. High-velocity blood flow in the feeding artery makes a difficulty in embolization of the aneurysm with AV fistula. Because deployments of coils in the arterial aneurysm itself have a high risk for coil migration through the fistula, the dilated feeding artery is selected to be embolized with large macrocoils or detachable coils. Unlike renal arterial aneurysms, reperfusion of the aneurysm will occur when AV shunt is remained. It is another technical difficulty in embolization of the vascular malformations that multiple coils are required for complete embolization of the feeding artery, because large microcoils exceeding AV fistula diameter can be passed through the AV fistula because microcoils are soft enough to be changed in shape. Accurate interpretation of the angiographic findings is needed to success TAE for the combined type AVM. The occlusion of cirsoid AVM in the combined type is essential. The use of alcohol for ablation of renal AVMs which can obliterate the core of the AVM has several advantages over other embolic agents [9]. In case 1, however, we did not use ethanol in the treatment of the AVM because there was a risk of major extravasation of ethanol from ruptured aneurysm, which cause the nerve palsy. In case 2, no epidural anesthesia which is needed for the ethanol ablation had been performed [5] because we had no idea of the possibility of the cisoid AVM before we obtained angiography. Instead, the feeding arteries and main renal arterial trunk were occluded by coil embolization which could not obliterate the core of the AVM, resulting that the occlusion of the venous aneurysm in the draining vein was fail. Instead of coils, n-butyl cyanoacrylate should be used in embolization of the renal AVM to occlude the venous aneurysm [10]. |
| CONCLUSION |
|
Both diagnostic and interventional radiologists should pay an attention of a new subtype of renal cirsoid AVM which is combined with aneurysm. |
| REFERENCES |
|
|
[HTML Abstract]
[PDF Full Text]
|
| Author Contributions
Toh Yamamoto – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Final approval of the version to be published Zenjiro Sekikawa – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Izumi Torimoto – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Shigeo Takebayashi – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of Submission
The corresponding author is the guarantor of submission. |
|
Source of Support
None |
|
Consent Statement
Written informed consent was obtained from the patient for publication of this case report. |
|
Conflict of Interest
Author declares no conflict of interest. |
|
Copyright
© 2018 Toh Yamamoto et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|