|
|
Case Series
| ||||||
| Two cases requiring nephrectomy in long-term follow-up of sporadic angiomyolipoma after transarterial ethanol ablation | ||||||
| Ryo Aoki1, Zenjiro Sekikawa2, Toshiaki Nishii3, Toh Yamamoto1, Yasuhide Miyoshi4, Shigeo Takebayashi5 | ||||||
|
1Department of Diagnostic Radiology (Resident), Yokohama City University Medical Center, Japan 2Department of Diagnostic Radiology (Assistant Professor), Yokohama City University Medical Center, Japan 3Department of Diagnostic Radiology (Attending Physician), Yokohama City University Medical Center, Japan 4Department of Urology (Associate Professor), Yokohama City University Medical Center, Japan 5Department of Diagnostic Radiology (Professor), Yokohama City University Medical Center, Japan | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article] [Similar article in PubMed] [Similar article in Google Scholar] |
| How to cite this article |
| Aoki R, Sekikawa Z, Nishii T, Yamamoto T, Miyoshi Y, Takebayashi S. Two cases requiring nephrectomy in long-term follow-up of sporadic angiomyolipoma after transarterial ethanol ablation. Edorium J Radiol 2018;4:100009R02RA2018. |
|
ABSTRACT
| ||||||
|
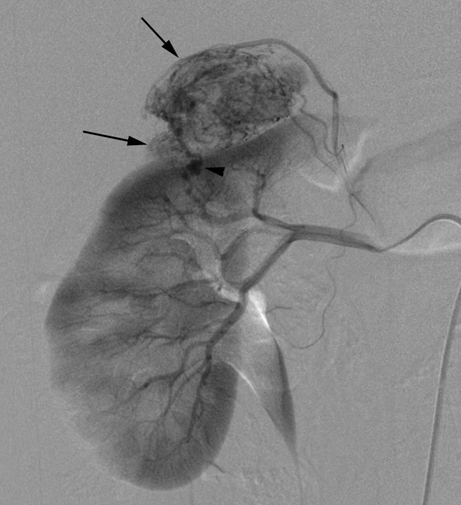
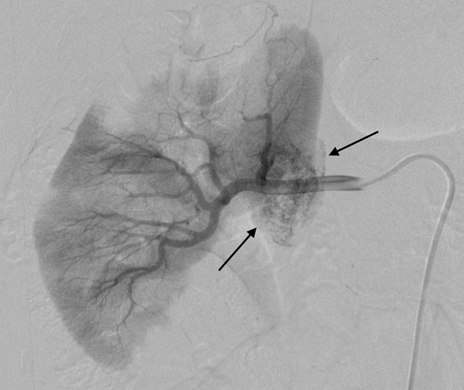
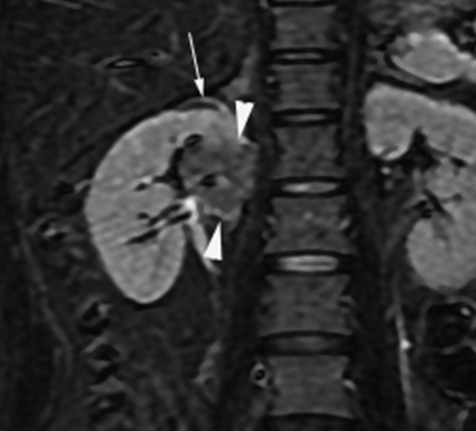
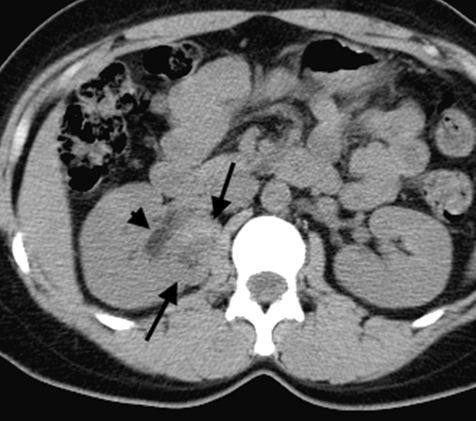
Introduction: We report two cases requiring nephrectomy in long-term follow-up of sporadic left renal angiomylipoma (AML) after transarterial ethanol ablation (TEA). Ethanol is an excellent sclerosing agent that causes complete occlusion of normal or abnormal renal arteries. Case Series: Case 1 was a 33-year-old woman with a large AML, 12 cm in diameter and concomitant hydronephrosis. A 60% volume reduction of the tumor was achieved by TEA on 1-year follow up. However, a laparoscopic left nephrectomy was performed due to a progressive hydronephrosis by a possible complication of the ablation of the ureteral artery as the volume of the AML was decreased. Pathological examination showed epithelioid AML in a part of the residual tumor and regional lymphnode involvement. Case 2 was a 42-year-old woman with two AMLs in the right kidney, a four cm-sized lesion in the upper pole and a two-cm lesion near the hilus. TEA was performed in the treatment of the AML in the upper pole. However, the one in the hilus was not treated because of potential risk for ablation of the ureteric artery. Nephrectomy was performed with a suspicious of left renal vein involvement of the enlargement of the untreated hilar AML on MDCT although a marked reduction of the volume of the AML in the upper pole on a 4-year follow-up was deserved. Pathological examination showed benign AML extending the renal sinus without the involvement of the renal vein. Conclusion: TEA can achieve a sufficient reduction of sporadic AML, but has a potential risk for residual tumor of malignant epithelioid AML. Additionally, the therapeutic technique has a fear of unintended ablation of the ureteral artery causing hydronephrosis. Keywords: Angiomyolipoma, Epitheliod angiomyolipoma, Ethanol ablation, Kidney | ||||||
|
INTRODUCTION
| ||||||
|
Renal angiomyolipoma (AML) is a mesenchymal neoplasm composed of a variable proportion of adipose tissue, spindle and epithelioid smooth muscle like cells, and abnormal thick-walled blood vessels. AMLs occurs sporadically within the general population in individuals without tuberous sclerosis and account for about 3% of all tumors in the kidneys and are found in < 0.3% of the general population [1]. Large sporadic AMLs, greater than 4 cm in diameter, require therapeutic intervention as prophylaxis against hemorrhage [2]. High rates of technical success which are complete intra-procedural occlusion of arterial inflow into the AML were reported to achieve in transarterial embolizations with various embolic agent or transarterial ablation with ethanol (99.9% alcohol) or admixture of ethanol and Lipiodol or Ethiodol. We have used ethanol which is an excellent sclerosing agent with liquidity and high occlusion potential for occlusion of arterial flows into AMLs [3]. Herein, we report two cases requiring nephrectomy in long-term follow-up of sporadic renal angiomylipoma (AML) after transarterial ethanol ablation (TEA) and discuss the usefulness and limitation of TEA in the management of sporadic AML. | ||||||
|
CASE SERIES
| ||||||
|
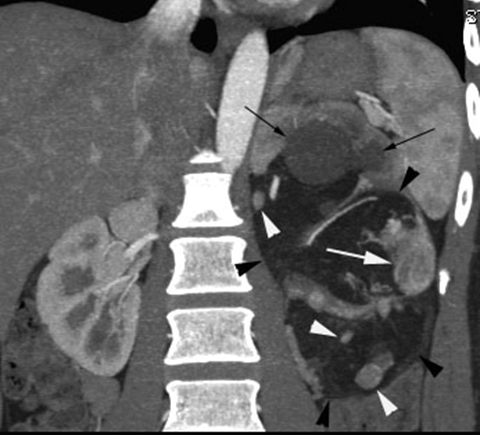
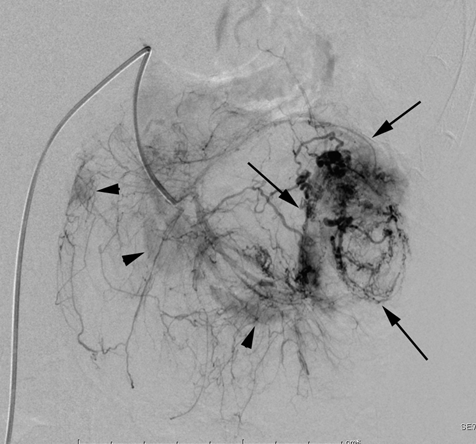
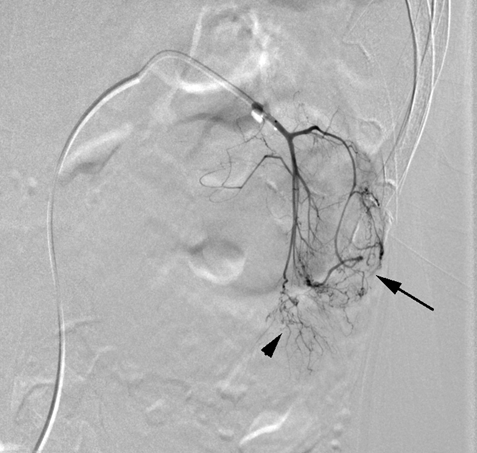
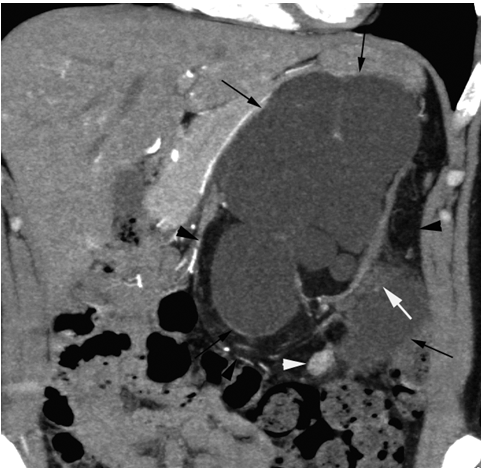
Case 1
Case 2
| ||||||
|
| ||||||
|
| ||||||
| ||||||
|
| ||||||
|
| ||||||
| ||||||
|
| ||||||
| ||||||
|
| ||||||
| ||||||
DISCUSSION | ||||||
|
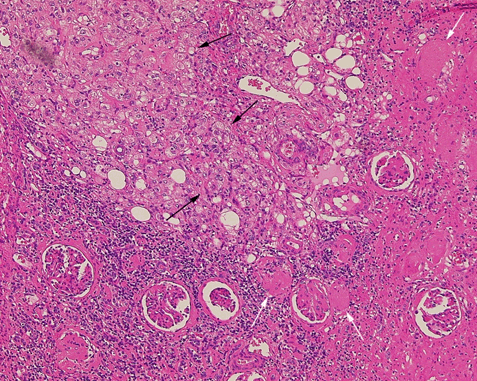
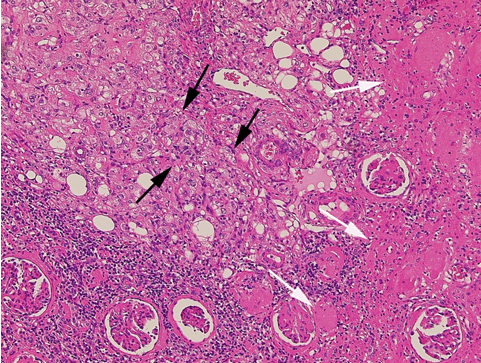
According to the review by Murray et al.[1] embolization or ablation for the treatment of AML was performed with ethanol in 41.7%, coil in 6.2%, gelatin sponge or microparticle in 5.2%. Absolute ethanol ablation which we used in our cases is superior to embolic agents in terms of the occlusion effect. Mean percentage areas of tumor reduction were 42.4 -70.8% in a 1-year follow-up in our previous reported series of TEA [3]. Over 90% volume of tumor reduction in 4-year follow up was achieved by TEA for benign AML in case 2. Ethanol ablation could also be achieved in a 60% volume of tumor reduction in 1-year follow up in case 1 with potential malignant epithelioid AML. Some authors have used ethanol mixed with contrast material to visualize the ethanol fluoroscopically. An ethanol-iodized oil mixture which is distinguishable from absolute ethanol ablation has also been used in the embolization of AMLs [4], [5]. Ethanol-iodized oil embolization is an alcohol based procedure in which the iodized oil is thought to act as an inert material although oily contrast material enhances the embolic effect of the alcohol by producing more homogeneous distribution of the agent and increasing its contact time with the tissue [6]. TAE has been a treatment option for hemorrhage and a prophylaxis for patients at high risk for bleeding. TAE can preserve the renal parenchyma in contrast to surgery. However, TAE of renal artery has a potential complication of hydronephrosis which is caused by the unintended ablation of the ureteral artery resulting perivascular ethanol extension to the ureter. The complication may occur in patient in whom the ureteral artery is not visualized on pre TAE angiography like case 1. The ureteral artery occasionally communicates directly with the segmental or interlobar branches without intermediary communication with exorenal arcade [7]. As epithelioid cellular morphology can be seen in clinically benign usual AMLs, Brimo et al. [8] categorized epithelioid AMLs with atypia into malignant potential. Atypical epithelioid cells were defined as atypical polygonal cells with abundant cytoplasm, vesicular nuclei, prominent nucleoli, and nuclear size that exceeds the size of adjacent nuclei. The radiological appearance of most epithelioid AMLs was reported to have a tendency to be hyper-attenuated on pre-contrast CT with or without fat component. CT with intravenous injection of contrast material shows a rapid or delayed washout pattern [9]. But those findings are non-specific for renal tumor. Additionally, benign AML increases in size with sub-centimeters per year [10] and can aggressively extend into the renal vein or regional lymphnode involvement [11], [12], [13]. Most patients with a large (> 4 cm in diameter) renal tumor with fat density is candidate for TEA or embolization in the treatment of AML. However, there remains a dilemma that most of the candidates who undergo no pathological examination which can exclude malignant epithelioid AML. Therefore, fine needle aspiration diagnosis is required and immunostaining of AML have challenged to improve diagnostic accuracy of epithelioid AML [14]. Surgery can be considered in a large AML such as case 1, and TEA or embolization may be required to diminish the dilated tumor vessels including aneurysms before the laparoscopic nephrectomy. | ||||||
|
CONCLUSION
| ||||||
|
TEA can achieve in a sufficient reduction of sporadic AML, but had a potential risk for residual tumor of malignant epithelioid AML. Additionally, the therapeutic technique had a fear of unintended ablation of the ureteral artery causing hydronephrosis. | ||||||
|
REFERENCES
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Ryo Aoki – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Final approval of the version to be published Zenjiro Sekikawa – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Toshiaki Nishii – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Toh Yamamoto – Acquisition of data, Critical revision of the article, Final approval of the version to be published Yasuhide Miyoshi – Acquisition of data, Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Shigeo Takebayashi – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of Submission
The corresponding author is the guarantor of submission. |
|
Source of Support
None |
|
Consent Statement
Written informed consent was obtained from the patient for publication of this case series. |
|
Conflict of Interest
Author declares no conflict of interest. |
|
Copyright
© 2018 Ryo Aoki et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|