|
|
Original Article
| ||||||
| Splenic artery embolization for traumatic and non-traumatic splenic injury | ||||||
| Zenjiro Sekikawa1, Toh Yamamoto1, Ryo Aoki1, Shintaro Furugori2, Shigeo Takebayashi1 | ||||||
|
1Departments of Diagnostic Radiology, Yokohama City University Medical Center, Yokohama, Japan 2Critical Care and Emergency Center (KN, NM), Yokohama City University, Medical Center, Yokohama, Japan | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article] [Similar article in PubMed] [Similar article in Google Scholar] |
| How to cite this article |
| Sekikawa Z, Yamamoto T, Aoki R, Furugori S, Takebayashi S. Splenic artery embolization for traumatic and non-traumatic splenic injury. Edorium J Radiol 2018;4:100008R02ZS2018. |
|
ABSTRACT
| ||||||
|
Aims:
The aim of this retrospective study is to identify clinical factors associated with the clinical outcome of both traumatic and non-traumatic patients who underwent splenic artery embolization (SAE) in the treatment of splenic hemorrhage.
Keywords: Embolization, Hemorrhage, Splenic artery, Trauma | ||||||
|
INTRODUCTION
| ||||||
|
The spleen is one of the most commonly injured abdominal organs after abdominal trauma [1]. Several decades ago, splenectomy was the sole treatment for traumatic splenic injury [2], leaving asplenic patients particularly vulnerable to infection with encapsulated organisms. Surgery still remains the gold standard for treating patients with splenic injuries with hemodynamic instability, and it has constituted up to 50% of cases [3]. Splenectomy is also the first choice for the treatment of atraumatic splenic injury. On the other hand, with blunt traumatic splenic injury, non-operative management has been employed as an alternative in hemodynamically stable patients [2],[4],[5], [6],[7],[8] and is becoming the new standard for treatment [9], not only for patients with abdominal multiorgan injuries [10] but also for children [11]. Among nonoperative approaches, transcatheter artery embolization (TAE) has been widely used to control bleeding in patients with abdominal injuries, as it can rapidly assure hemostasis. In 1981, Sclafani presented 4 patients with splenic injuries, in whom angiography and splenic artery embolization (SAE) were applied [12]. After that, many studies have confirmed the effectiveness of SAE in hemodynamically stable patients with blunt traumatic splenic injuries, showing that SAE was able to increase the success rate of non-operative management [13],[14]. Criteria for non-operative management includes 1) the restoration of hemodynamic stability with minimal fluid resuscitation; and 2) the absence of significant associated injuries requiring surgical intervention. However, controversy remains regarding the indications for SAE [1],[5],[6],[8],[14],[15]. Moreover, a number of studies have suggested that various clinical factors should be used to guide the choice of treatment modality but still fail to reach any conclusions [16],[17]. SAE for patients with non-traumatic splenic injury has also not yet been established with definite value. We retrospectively reviewed the medical records of patients with traumatic splenic injury and non-traumatic splenic injury who received SAE, compared the factors of both groups and assessed the outcome of SAE to clarify clinical factors that are associated with clinical outcome of the patients who underwent this procedure. Infective endocarditis is defined as pathological evidence of microorganisms demonstrated by culture or histological examination of a vegetation, a vegetation that has embolized or intracardiac abscess alongside clinical evidence proposed by Modified Duke Criteria [1]. It is related to 40,000 to 50,000 new cases each year in the United States [2], being the most worrisome aspect, the increasing trend in health care associated factors, such as in hemodialysis patients [3], [4]. Staphylococcus aureus has been described as the most common causative organism of health care associated endocarditis including hemodialysis patients [1],[2], [3], [4], [5]. In the last decade and so, there are some cases reported [6],[7] among patients on hemodialysis with long term tunneled central venous catheters with associated fibrin sheath formation leading to a non-valvular source of infective endocarditis. The most common presentation of this entity is the formation of fibrin sheath vegetation in the superior vena cava [7],[8], that can be overlooked by transthoracic echocardiography (TTE) [6],[7],[9]. Thus, transesophageal echocardiography (TEE) has been suggested as the initial diagnostic approach in such cases [6],[7],[8],[9]. We report a case of a young female patient with end stage kidney disease on hemodialysis who presents with progressive dyspnea associated to a flu like illness who was initially admitted under the presumptive diagnosis of community acquired pneumonia and was later diagnosed with infective endocarditis secondary to a fibrin sheath vegetation. | ||||||
|
MATERIALS AND METHODS
| ||||||
|
This retrospective study obtained the approval of our institutional review board. We confirmed that the patients or the legal representatives of the patients in this study were given a comprehensive written statement of information about the clinical study, including information on SAE, and their consent was documented in the clinical records. We reviewed the records of 84 consecutive patients who were admitted to the Emergency Center of Yokohama City University between January 1996 and April 2015 for blunt splenic injuries with or without injuries to other organs. Patients who required emergency surgery for gastrointestinal tract injury or those with severe hemodynamic instability did not undergo angiography. Those patients with stable hemodynamics, implying a lack of significant bleeding, also did not undergo angiography. Thus, 49 of 84 patients underwent angiography. Of these 49 patients, four patients had an injury grade that could not be classified due to incomplete documentation in the clinical chart, and those patients were excluded from the study. An additional two patients were also excluded from the study; in one case, TAE was performed to stop pancreatic hemorrhage after splenectomy, and in the other case, the patient underwent SAE for delayed splenic rupture after a period of conservative management in another hospital. The remaining 43 patients (33 males and 10 females) were included in this study. The patient age range was 8–77 years (mean±SD, 37.6±19.1). Inciting events for splenic injury included traffic accidents (n=27), falls (n=11), and assaults (n=3). Two other cases included hit by falling down and uncertain origin. Additionally, we reviewed the records of 15 consecutive patients who were admitted to our institution for non-traumatic splenic injuries and received SAE. One patient who was actually bleeding from pancreatic artery was excluded from the study. The remaining 14 patients (ten males and four females) were included in this study. The patient age range was 41–80 years (67.4±9.4). Inciting events for non-traumatic splenic injury included rupture of a splenic artery aneurysm, vascular malformation, neoplasm, and spontaneous bleeding. Radiologists examined all 57 patients using standard angiographic techniques as shown later in detail. Those radiologists were well trained, board-certified, and had more than eight years of SAE experience in the emergency department. The decision to perform embolization was ultimately made by those radiologists. Indications usually included the presence of extravasation or pseudoaneurysm. Even if there was no evidence of extravasation, patients proceeded to SAE if they had evidence of disruption of terminal arteries or avascularity and irregularity in the accumulation of contrast medium. A good clinical outcome was defined as the ability to control bleeding successfully without the use of ancillary methods. A poor clinical outcome of patients was defined as inadequate hemostasis as documented by ultrasound (an expanding collection), intraoperative observations (visual bleeding), clinical scenario (hemodynamic instability despite of continuous blood transfusion) (n=6), and death within 6 hours of SAE (n=4).
Splenic artery embolization
| ||||||
|
RESULTS
| ||||||
|
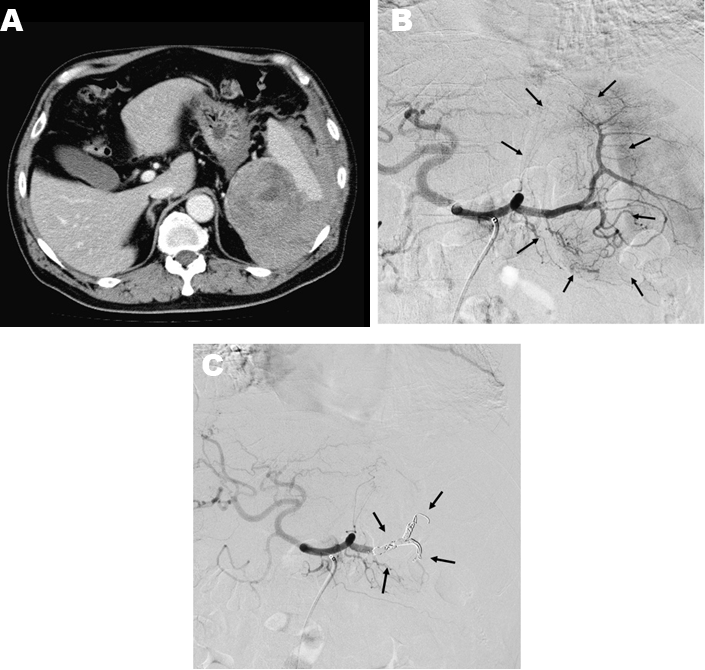
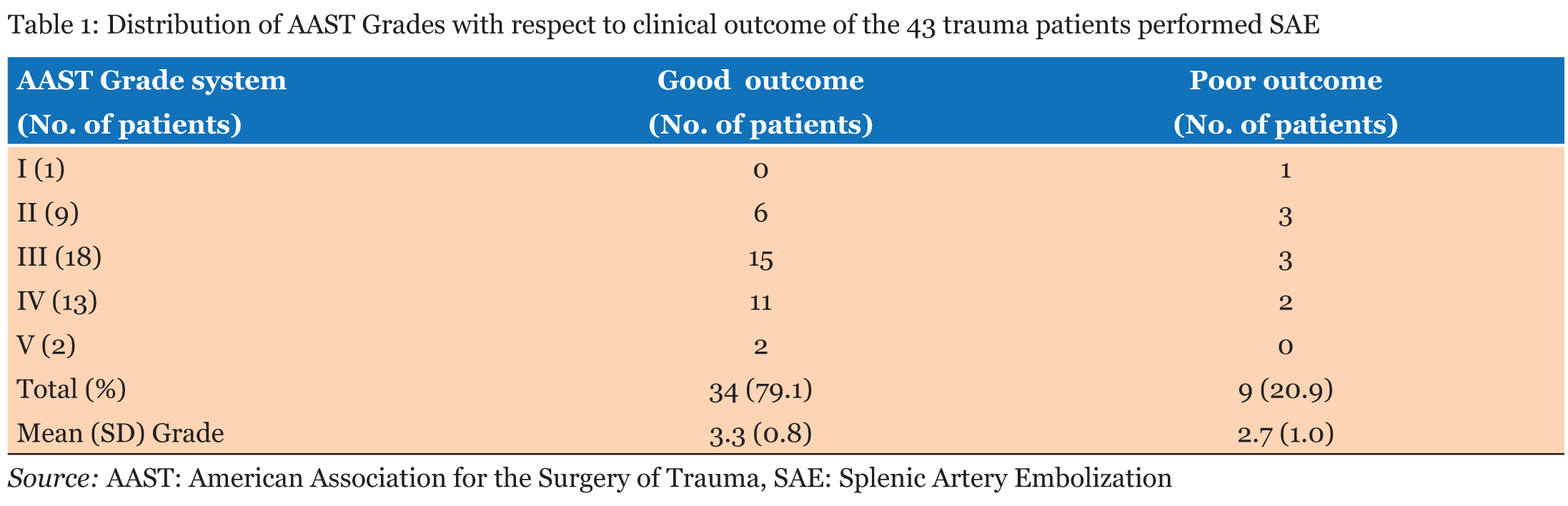
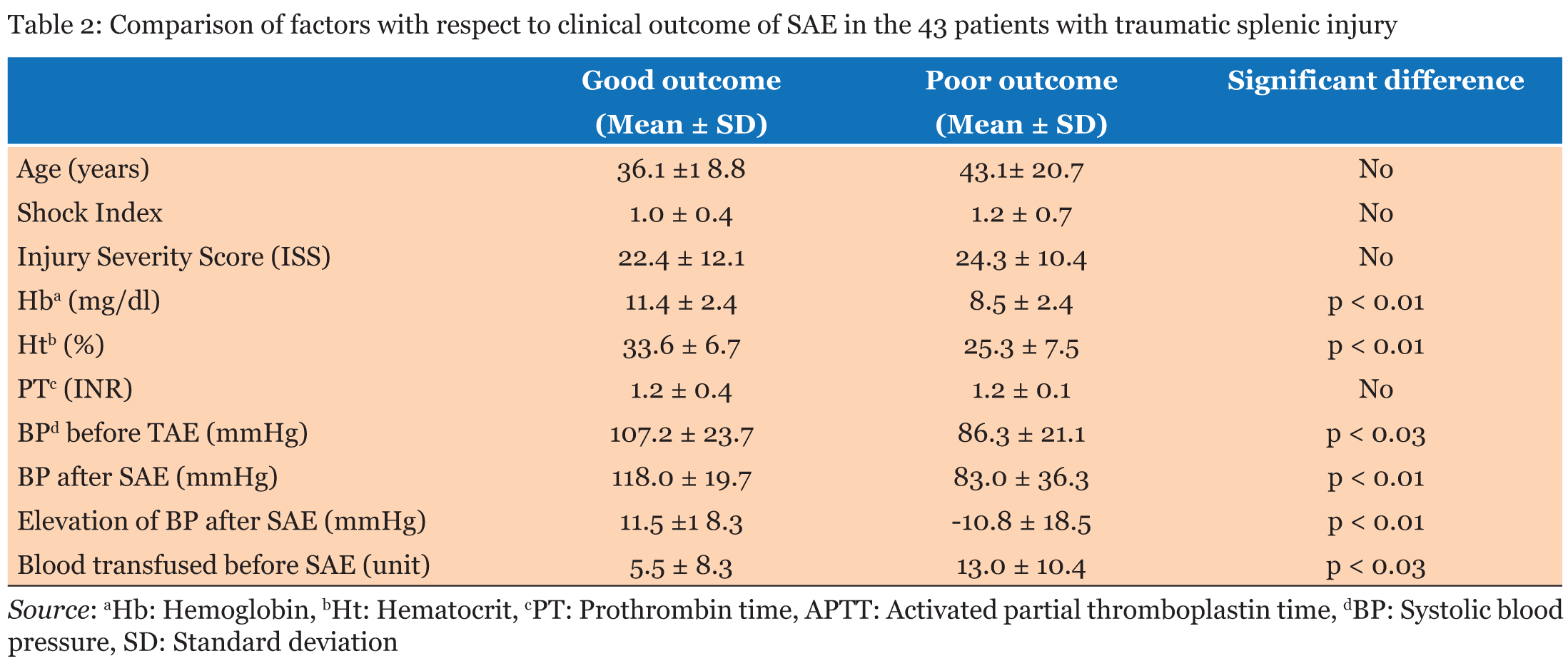
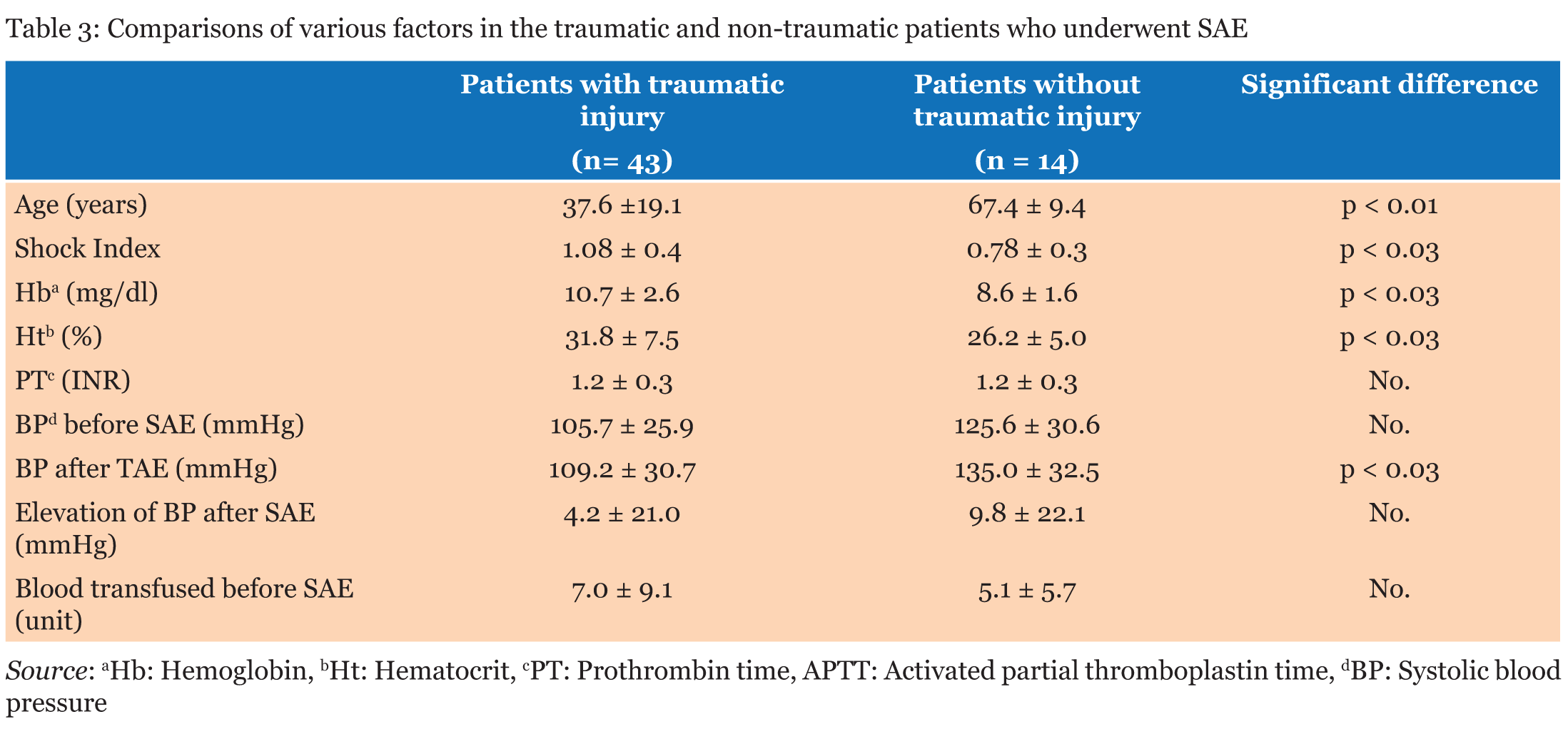
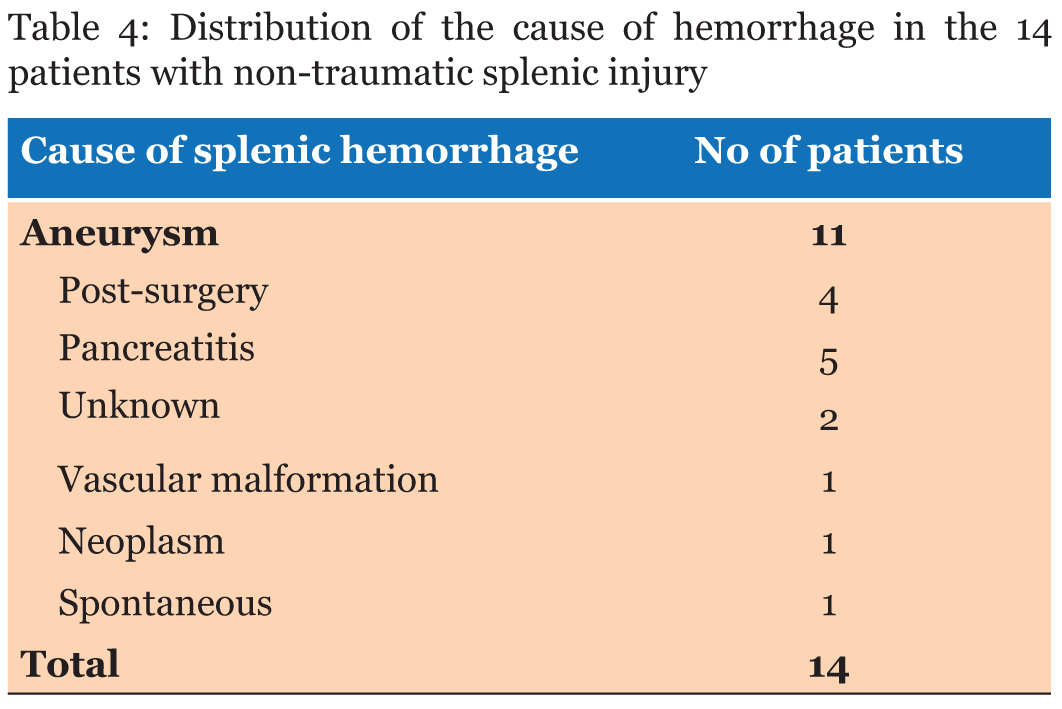
Of the 45 patients with blunt splenic injury that underwent TAE, 43 qualified for this study. Of these 43 patients, we confirmed good clinical outcome in 34 patients (79.1%). Grade of injury was not a significant predictor of clinical outcome (Table 1). There was no significant association between patient age and clinical outcome or between SI (0.44 to 2.50), ISS (4.0 to 50.0) and clinical outcome (Table 2). Lower values of Hb and Ht were significantly associated with poor clinical outcome (Table 2), but differences in PT were not (Table 2). BP range was 50-161 mm Hg just before SAE and 40-170 mm Hg after SAE. BP elevation during SAE ranged from -50 to 70 mm Hg. Lower values of each of these parameters were significantly associated with poor clinical outcome (Table 2). Blood transfusion requirements before SAE (range, 0–40 units) were significantly associated with clinical outcome of the patients (Table 2), with lower blood transfusion requirements associated with a favorable clinical outcome. In the non-traumatic group, mean age was significantly higher than that of the traumatic group, SI, Hb, and Ht were lower, and post-SAE BP was higher (Table 3). Inciting events for non-traumatic splenic injury included rupture of splenic artery aneurysm (n=11), vascular malformation (n=1), neoplasm (n=1), and spontaneous bleeding (n=1) (Table 4) (Figure 1). One case with non-traumatic splenic injury in which SAE failed showed a trend for lower Hb (4.9 vs 8.6±1.6: average), lower Ht (14.2 vs 26.2±5.0), prolonged PT (2.1 vs 1.2±0.3), and lower BP (76 vs 125.6±30.6), but no statistical analysis was performed because this was the only case with failure of SAE in non-traumatic splenic injury. | ||||||
|
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
DISCUSSION
| ||||||
|
Several decades ago, splenectomy was the sole treatment of choice for the patients with blunt splenic injuries. Over the last decade, non-operative management has become the preferable treatment for hemodynamically stable patients with blunt splenic injuries and the failure rate of the non-operative management has considerably decreased, possibly attributed to the introduction of SAE. A number of studies have previously assessed the success rate of SAE for the management of bleeding in blunt splenic injury. In this series, we confirmed good clinical outcome in 79.1% of patients, which is slightly lower than previously reported success rates of over 80% [4], [5], [6] . Considering the wide indications for SAE (such as including patients with multiorgan injuries) and strict definition of good clinical outcome in the present study, these factors might contribute to the reduced success rate, and we were able to attain a considerably favorable outcome. Mean ISS in our study (22.8±11.7) were slightly higher than those in previous reports [4], [5], [6] . For example, Sclafani et al. reported a success rate of 95%, but patients in their study had a mean ISS of 18, and the rate of associated complications such as pelvic injury was lower than that in the present study (4/60<0.1% vs. 12/43=27.9%) [4]. A large analysis that included 23,532 patients with blunt splenic injuries showed that the frequency of non-operative management failure was proportional to a higher AAST grade and ISS [18]. Another study of 6,308 patients with blunt splenic injuries showed the frequency of unsuccessful non-operative management were associated with the AAST grade and the amount of intraperitoneal hematoma [19]. Brasel et al. reported that injury grade was the only factor related to the success rate of non-operative management [7]. In the present study, splenic injury was distributed between all AAST grades (I-V), but injury grade did not emerge as a significant factor influencing the clinical outcome of SAE. Additionally, ISS did not affect the outcome. These results are contrary to previous reports supporting the influence of higher AAST grade and higher ISS on the higher failure rate of non-operative management. Reasons for this discrepancy may include the backgrounds of the patients or design of the present study. Indeed, Moore et al. noted that the AAST splenic injury scale was not developed to assign a prognostic value [20]. In grading splenic injury, we used the established practice of computed tomography (CT). However, there were some reports indicating CT findings often show no correlation to the severity of the splenic injury [21],[22] and were notoriously poor in identifying vascular injuries [4], [5]. For example, Sutyak et al. reported that CT findings were a poor predictor of operative findings of the degree of adult splenic injury [21]. However, currently CT examinations have been thought essential for the choice of treatment in patients with splenic injuries [6],[14],[15],[23]. The results presented by the National Trauma Registry of the American College of Surgeons showed contrast CT blush was one of the factors in non-operative management failure [14]. The results of other studies also have shown that the presence of contrast blush on CT was correlated with extravasation on angiography, and was correlated with a definitely higher risk of non-operative management failure [6],[23]. Bhullar et al. found a strong correlation between the presence of contrast blush found on CT and active bleeding found on angiography [15]. They also emphasized that CT contrast blush indicated the necessity of applying embolization in patients with blunt splenic injuries who qualified for non-operative management. Good clinical outcome was independent of patient age. Smith et al. suggested that patients over 55 years of age require surgical management [2], while Brasel et al. concluded that there was no correlation between age and the success rate of nonsurgical management [7]. The concept that patient age does not affect the success rate of non-operative management seems to have prevailed recently [7], but some studies indicate that aging is one of the predictors for the failure of non-operative management [24]. Previous studies have demonstrated that operative management of patients with blunt splenic injury was employed for patients with significantly higher ISS than for those managed nonoperatively [6],[21]. Velmahos et al. compared the ISS of patients with positive angiograms to those with negative angiograms and found no significant difference [8]. Davis et al. reported that a higher ISS does not automatically predict failure of non-operative management [6]. Olthof et al. undertook a systematic review of studies to identify prognostic factors for non-operative management failure in patients with blunt splenic injuries. The severity of injury according to the ISS, with ISS>25, is one of the factors predicting non-operative management failure [24]. In the present study, mean SI and mean ISS had no significant influence on the clinical outcome of the patients with blunt splenic injury. Although it is reasonable to expect that low Hb, low Ht, impairment of the coagulation system and low blood pressure would contribute to poor clinical outcome, there is no definite data to confirm this supposition. In the present study, we demonstrated that low Hb, low Ht, and low blood pressure were associated with poor clinical outcome of the patients with blunt splenic injury who underwent SAE. However, impairments in coagulation (PT) showed no association with clinical outcome. As to non-traumatic splenic injuries, most of the cases involved rupture of splenic artery pseudoaneurysms, which were associated with postoperative inflammatory changes around the surgical site, such as inflammation of the pancreas, stomach, colon and other causes of pancreatitis. A tumor could also be the cause of atraumatic splenic rupture. Splenectomy is the first choice for treatment of atraumatic splenic rupture. A study of atraumatic splenic rupture found that even if all preconditions for non-operative management are met, the failure rate is high [25]. It also states that even in hemodynamically stable patients, there are three reasons splenectomy is chosen as follows 1. Histological examination of the spleen will establish the etiology of the atraumatic splenic rupture as well as any underlying systemic diseases. 2. A significant number of malignant diseases may cause atraumatic splenic rupture, so any organ-preserving approach should be prohibited. 3. The splenic function might already be compromised by a pathological alteration or infiltration of the splenic parenchyma, and under such a hyposplenic condition, removal of the non-functioning spleen is justified and will not increase the risk of an overwhelming postsplenectomy infection. We performed SAE first even for non-traumatic patients if they were included in the category of non-operative management. Instead of splenectomy, SAE can be an alternative option to improve hemodynamic condition in either trauma or non-trauma patients with splenic hemorrhage. However, this approach should be used if the interventional radiology team has the appropriate knowledge and experience to perform SAE. Matsumura et al. reported two cases of atraumatic splenic rupture that received splenic artery occlusion before splenectomy as well [26]. Schnüriger et al. found no significant difference in major complications, such as requiring splenectomy after SAE between proximal and distal embolization, but minor complications, such as minor infarctions, were significantly more frequent after distal embolization [27]. Concerns exist regarding the remaining splenic function after embolization. A small study comparing 15 previously embolized patients, 14 splenectomy patients, and 30 control subjects showed both embolized and splenectomy patients had higher leukocyte and platelet counts compared to controls. It also showed that there was no significant difference in the size of the spleen or immunoglobulin titers between embolized patients and controls [28]. A Japanese study reported on immunologic alterations after splenic preservation such as embolization or splenorrhaphy compared to those who underwent splenectomy, and it showed no discernible advantage to preservation over splenectomy [29]. These results quite engage our interest, and the immunologic effects after SAE still remains unclear and needs to be discussed further. We retrospectively reviewed the medical records of 84 patients with blunt splenic injury and assessed 43 patients in regards to SAE clinical outcome. Low Hb, low Ht, low blood pressure before and after SAE, decreases in blood pressure during the procedure, and increased transfusion requirements before SAE were all associated with poor clinical outcome. Injury grade, patient age, SI, ISS, PT did not significantly affect the clinical outcome of the patients who underwent SAE in blunt splenic injury. | ||||||
|
CONCLUSION
| ||||||
|
Non-traumatic splenic hemorrhage occurs mostly in patients with rupture of splenic artery pseudoaneurysm; however, other rare cases such as malignant tumors should be taken into account. Patients with non-traumatic splenic injury tend to be older in age and have lower Hb and lower Ht, but the result of SAE was considerably favorable. These results may indicate the treatment of choice in patients with traumatic and non-traumatic splenic injuries. More prospective, randomized studies are still required. | ||||||
|
REFERENCES
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Zenjiro Sekikawa – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Toh Yamamoto – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Ryo Aoki – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Final approval of the version to be published Shintaro Furugori – Substantial contributions to conception and design, Drafting the article, Final approval of the version to be published Shigeo Takebayashi – Substantial contributions to conception and design, Acquisition of data, Drafting the article, Final approval of the version to be published |
|
Guarantor of Submission
The corresponding author is the guarantor of submission. |
|
Source of Support
None |
|
Consent Statement
Written informed consent was obtained from the patient for publication of this study. |
|
Conflict of Interest
Author declares no conflict of interest. |
|
Copyright
© 2018 Zenjiro Sekikawa et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|