| Table of Contents |  |
|
Original Article
| ||||||
| Non-inflammatory or non-ischemic vascular gas on emergent multi-detector computed tomography: Eight years' experience | ||||||
| Kazuya Sugimori1, Izumi Torimoto1, Kyota Nakamura1, Masaaki Kondo1, Kazushi Numata1, Shigeo Takebayashi1 | ||||||
|
1From the Gastroenterology Center (KS, MK, KN), the Department of Diagnostic Radiology (IT, ST) and, the Critical Care and Emergency Center (KN), Yokohama City University Medical Center.
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Sugimori K, Torimoto I, Nakamura K, Kondo M, Numata K, Takebayashi S. Non-inflammatory or non-ischemic vascular gas on emergent multi-detector computed tomography: Eight years' experience. Edorium J Radiol 2016;2:12–19. |
|
Abstract
|
|
The study aimed to characterize the etiology and clinical significance of non-inflammatory or non-ischemic vascular gas on multi-detector computed tomography (MDCT). We reviewed MDCT images and clinical charts of patients with vascular gas excluding inflammatory or ischemic entities in our hospital between 2008 and 2015. The local cases and the case report papers, which were extracted from English literature in PubMed were summarized according to iatrogenic or non-iatrogenic causes to analyze etiology for the entry of air into the circulation.Our local series demonstrated single or multiple collection of vascular gas in 15 patients including one with systemic arterial gas; the most frequent was cerebral vascular gas (CVG, n = 11, 0.8–12 mL) followed by hepatic vascular gas (n = 10, 0.4–256 mL). The accumulative 144 cases including the 15 local cases included 62 (43.1%) with iatrogenic vascular gas; the most frequent was central venous catheter-related CVG (48 cases) with 39.5% mortality followed by hepatic portal venous gas (20 cases) with 15% mortality. A careful search for clues on MDCT images was useful in discussing the etiology of vascular gas entry points and increased awareness of the emergent clinical settings where the vascular gas occurred.
| |
|
Keywords:
Brain, Iatrogenic complication, Portal vein, Vascular gas
| |
|
Introduction
| ||||||
|
Multi-detector computed tomography (MDCT), which allows a thin-slice scan and has been used emergently in patients in various clinical settings, is useful in the detection of small amounts of vascular gas. A post-processing algorithm using MDCT data that can generate multiplanar images allows assessment of anatomical location of vascular gas [1]. Unlike arterial gas, small venous gas produces no clinical signs and symptoms. However, the embolism can easily occur when gas enters the cerebral vein because the air blood interaction causes the development of a network comprising air bubbles and fibrin strands that intersperses with aggregates of the platelets, red blood cells, and fat globules [2]. We encountered vascular gas in various clinical situations, including iatrogenic complications. The morbidity and mortality associated with vascular gas resulting in embolism can be prevented through a better understanding of its pathophysiology and increased awareness of various clinical settings where they occur. Emergency departments are especially high-risk locations for venous air embolism because of frequent use of venous access (central and peripheral) and intravenous drug and fluid infusions [3]. We present this article analyzing the etiology of air entry into the veins on emergent MDCT scanning in our experienced cases as well as extracted published cases by systematic literature reviews. | ||||||
|
Materials and Methods
| ||||||
|
Approval for this retrospective study was obtained from our institutional review board, and a waiver for informed consent was obtained. Local cases Literature search | ||||||
|
Results | ||||||
|
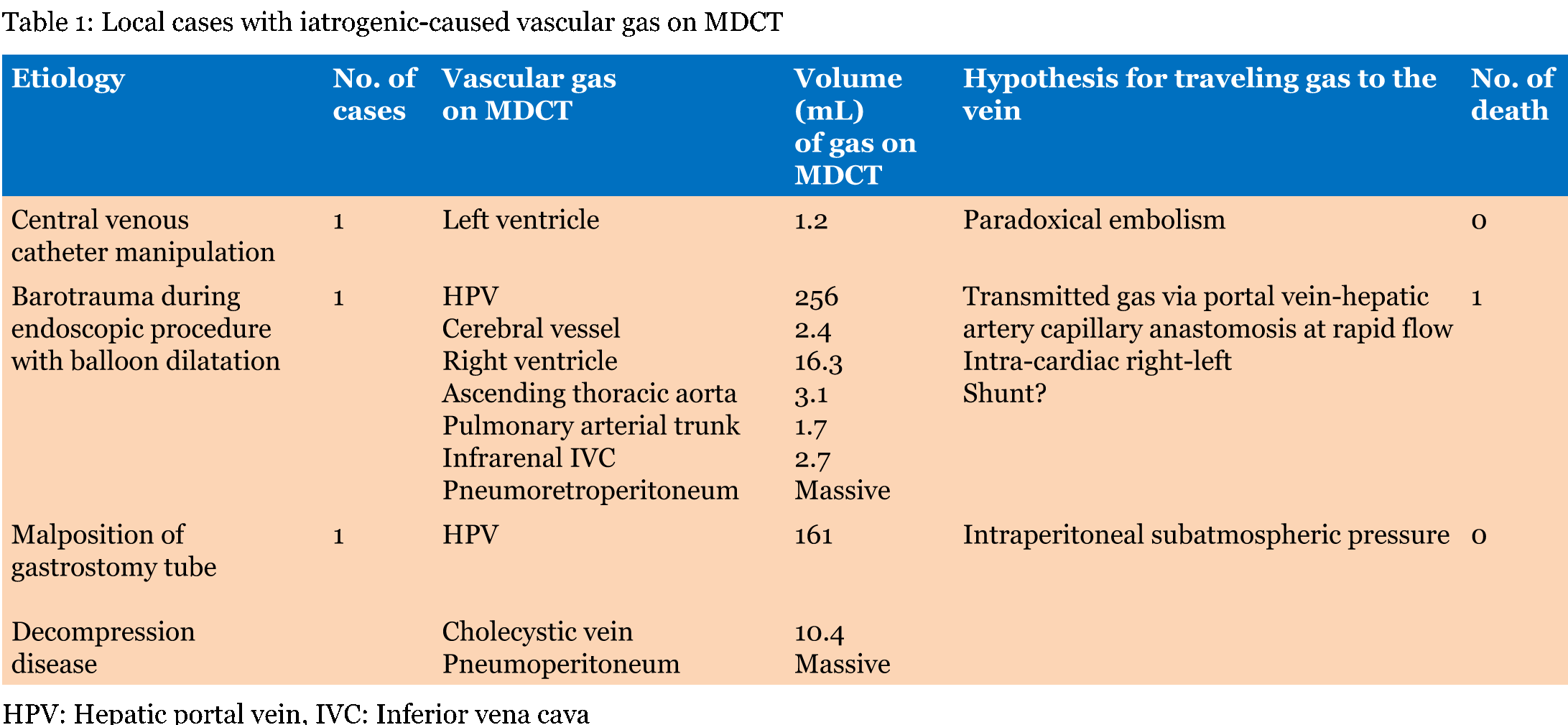
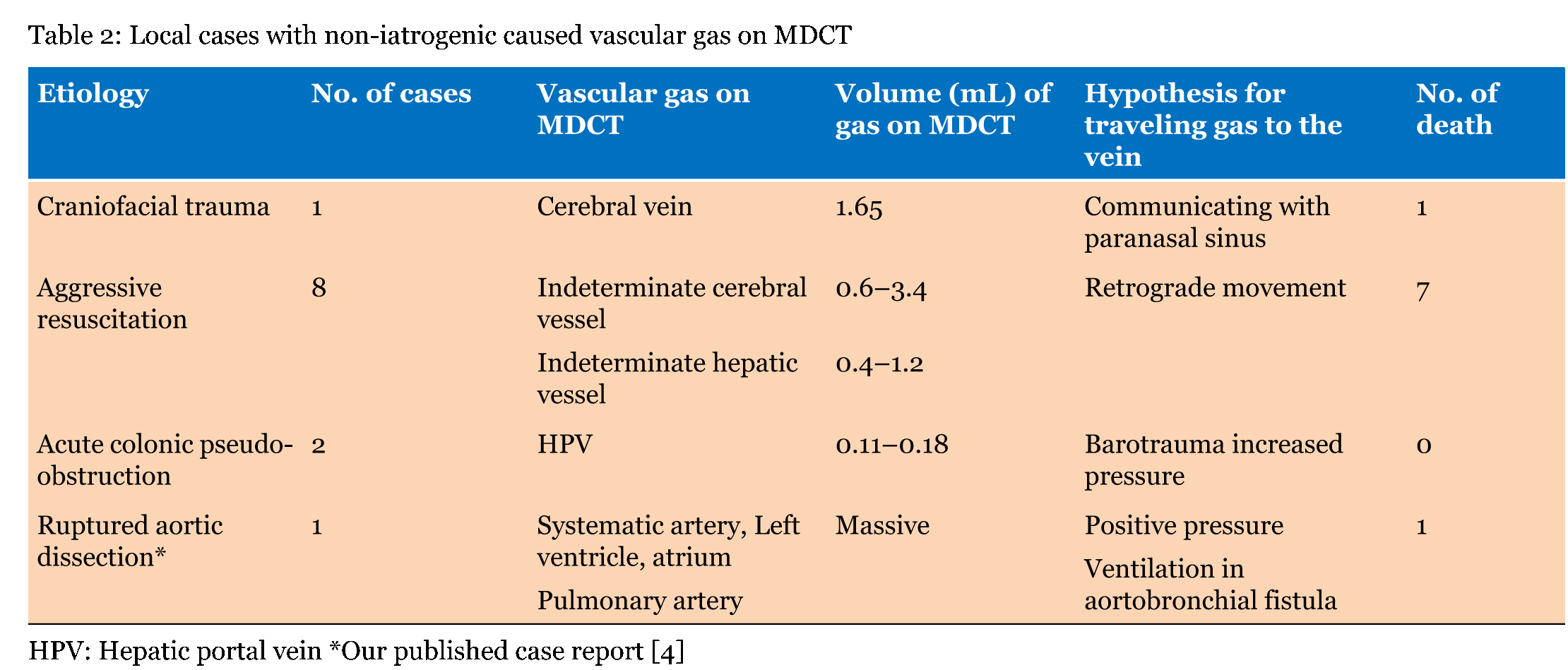
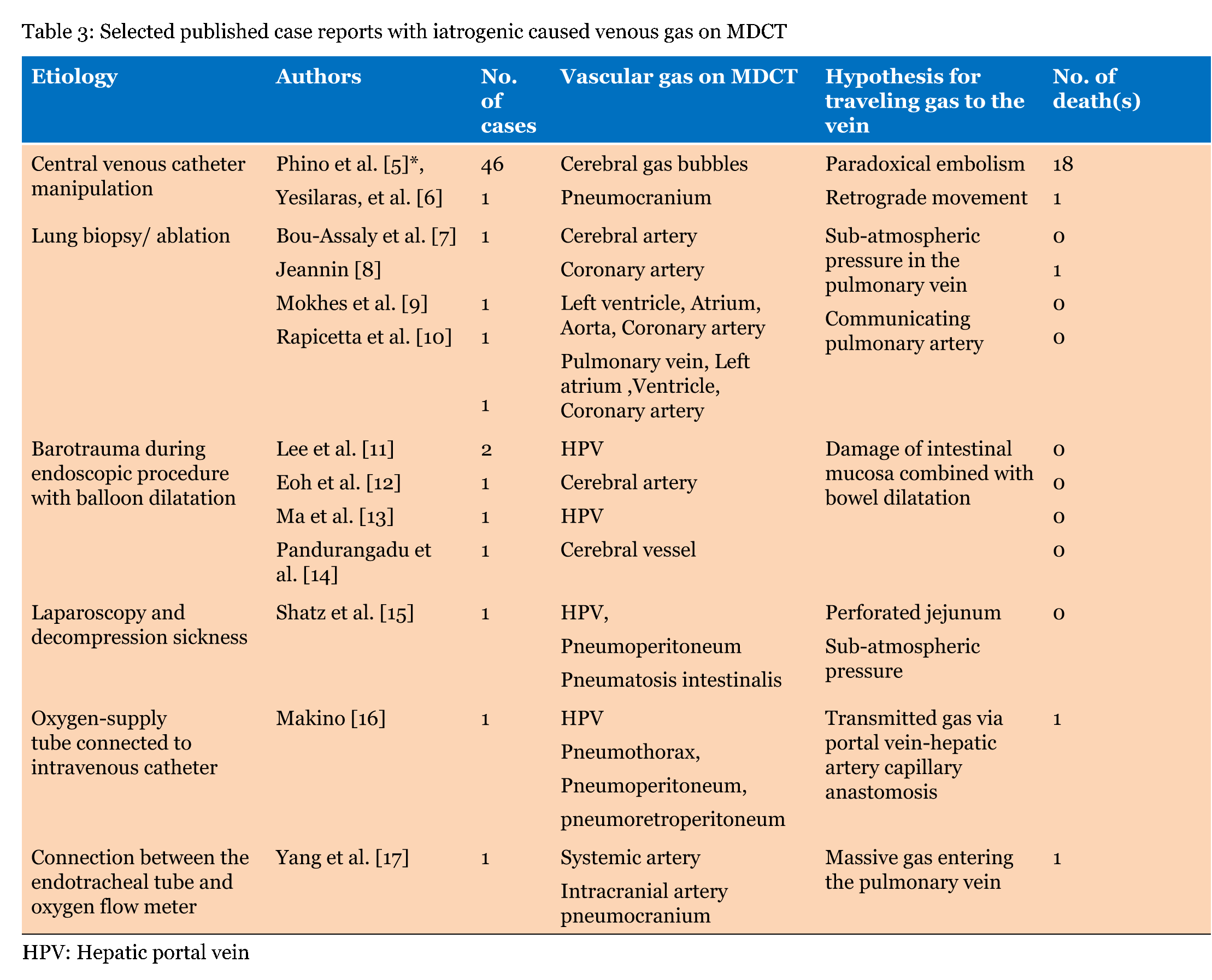
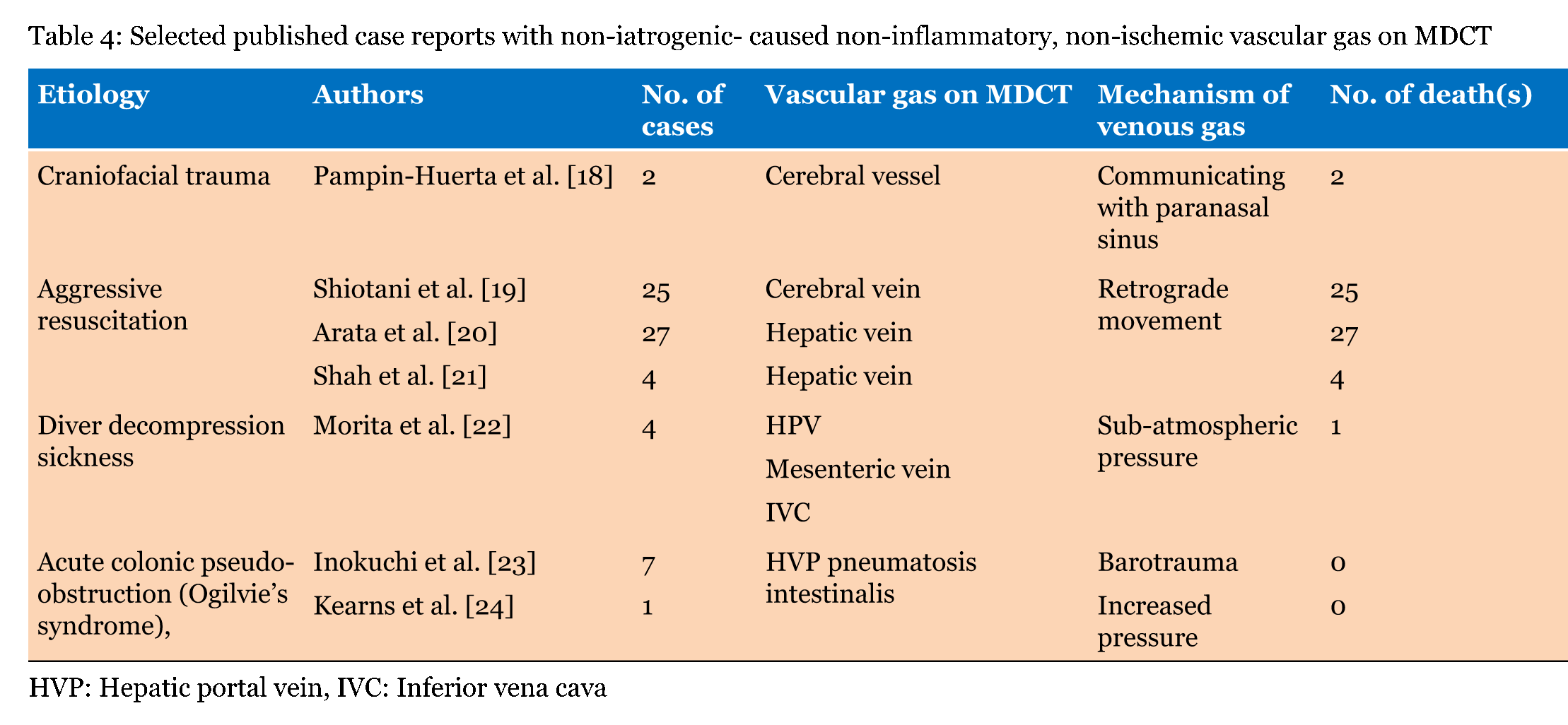
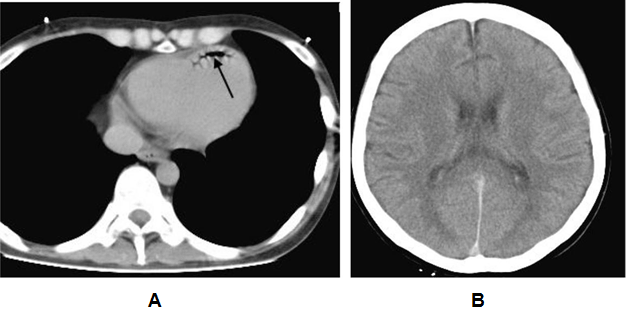
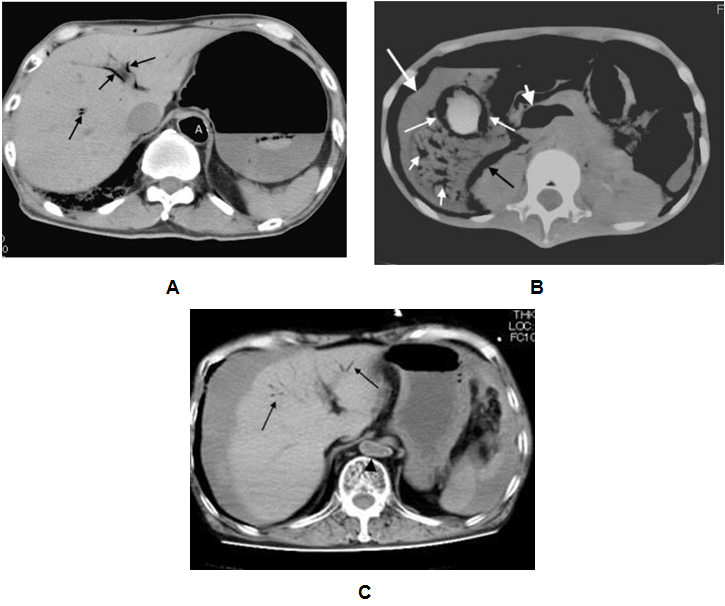
Our local series demonstrated single or multiple collections of vascular gas in 15 patients who were three with iatrogenic vascular gas (Table 1) and 12 with non-iatrogenic gas (Table 2); the most frequent was cerebral vascular gas (CVG, n = 11, 0.8–12 mL) followed by hepatic vascular gas (n = 10, 0.4–256 mL). A total of 20 case reports, including one review article with 165 included cases, were extracted by PubMed search terms related to vascular gas in combination with "cerebral" or "hepatic". The majority of literature reported on single patients. MDCT or computed tomography scan was performed in 129 of the 165 patients. We selected the 129 patients, including 59 with iatrogenic-caused vascular gas (Table 3) and 70 with non-iatrogenic-caused vascular gas (Table 4). Of the accumulative 144 cases which were 129 published reporting cases and 15 local cases, 62 (43.1%) including 3 local cases were iatrogenic and 82 (56.9%) including 12 local cases were non-iatrogenic. The most frequent entity of iatrogenic vascular gas in the central venous catheter-related CVG (48 cases, 77.4%) with 39.5% mortality. Our local series had a patient with CVG-related cerebral embolism in which no intracranial gas but 1.2 mL of the left ventricular gas was shown in Figure 1. In this patient, the presence of the left ventricular gas supported the paradoxical embolism although no intra-cardiac right to left shunt was shown in the color Doppler imaging. In the local case series, massive (>100 mL) hepatic portal venous gas was observed in the remaining two patients with iatrogenic complications. The patient experienced cardiac arrest during the endoscopic procedure with balloon dilatation. Post-resuscitation MDCT images Figure 2 demonstrated multiple collections of gas in the right ventricle, the pulmonary arterial trunk, infrarenal inferior vena cava, the ascending thoracic aorta and cerebral vessels. The barotrauma during the endoscopic procedure with balloon dilatation for bowel is a possible entity of massive hepatic portal venous gas, according to four published reporting cases. Massive hepatic portal venous gas associated with cystic venous gas in another local case occurred due to decompression sickness with sub-atmospheric intraperitoneal pressure and the entry of gas into injured gastric wall vein via a mal-positioned gastrostomy tube (Figure 3B) . One published case reported massive hepatic portal venous gas in decompression sickness with injured jejunum. Other etiologies of iatrogenic cerebral gas in published cases included percutaneous lung biopsy or radiofrequency ablation. Aggressive resuscitation was the most frequent (66.7%, n = 8) cause of vascular gas in non-iatrogenic local cases: intracranial gas in three patients, hepatic vascular gas in three patients and both in two patients. MDCT images did not provide the conclusive evidence of cerebral or hepatic vascular anatomies. MDCT images of craniofacial trauma in the local series showed gas in the overt cerebral vein (Figure 4C). Non-iatrogenic hepatic portal venous gas occurred in patients with acute colonic pseudo-obstruction in two local cases. The local case series included gas replacement of systemic arteries caused by positive pressure ventilation in the patient with aortobronchial fistula secondary to the aneurysmal rupture. MDCT in the patient showed conclusive images of intrahepatic arterial gas (Figure 3A) and cerebral arterial gas (Figure 4A-B). | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
Discussion
| ||||||
|
The entry of gas into the circulation is mostly an iatrogenic problem but may also occur in many fields of medicine including emergency medicine. The entry of gas into the blood stream requires a pressure gradient favoring the passage of gas into the blood vessel. This occurs when venous pressure is negative relative to atmospheric pressure or when gas is forced under pressure directly or indirectly into the bloodstream [3] [16]. A 14 G catheter enables the gas to flow at a rate of about 100 mL/sec, with a pressure difference of only 5 cm H2O [16]. Entering gas into the circulation occurs by barotrauma caused by positive pressure ventilation and insufflation of gas into the peritoneal cavity during endoscopy can also cause gas to enter the circulation [13]. The most frequently iatrogenic vascular gas was central venous catheter-related cerebral gas embolisms. Approximately, 70% of the patients with gas embolisms had intracranial gas bubbles, which were most frequently located in the subarachnoid space [5]. MDCT provides no conclusive anatomy with regards to the gas in the subarachnoid space although it can depict a small gas bubble. Therefore, the gas bubble was interpreted by some authors as intra-arterial caused by paradoxical embolism and by others as intra-venous caused by retrograde embolism [6]. Hagen et al. [25] demonstrated in a large number of autopsy specimens of human hearts that 27.3% had foramen ovales of 1 to 10 mm in diameter. Increasing right atrium pressure from entering gas is suggested to cause right to left shunting in the fossa ovale which is usually occluded [26]. In the majority of published cases, there was no reference to shunt investigation. The presence of the intra-cardiac right-left shunting on transesophageal echocardiogram is not always shown in patients with paradoxical embolisms. In a local case with central venous catheter trouble, the presence of left ventricular gas supported a paradoxical embolism, although no intra-cardiac right to left shunt was shown in the echocardiogram. Paradoxical arterial embolism is an acceptable mechanism because many cases reported central venous catheter-related coronary arterial gas embolism. But Yeslaris et al. [6] claimed that retrograde gas embolism possibly occurred through the internal jugular vein into intracranial veins when massive amounts of air entered the jugular vein via a large bored central venous catheter. Retrograde gas movement through the internal jugular vein to the intracranial venous sinus is considered as the mechanism of post-resuscitation cerebral gas or hepatic gas [20] [21] Post-resuscitation cerebral and hepatic gas are explained by the retrograde gas movements; at rapid infusion of large fluid volumes, contaminated air via the jugular or femoral central venous catheter is allow to enter the cerebral vein or hepatic vein, respectively [21]. Hepatic portal venous gas has been described in non-obstructive dilatation of bowels in acute colonic pseudo-obstruction (Ogilvie's syndrome) and Crohn's disease [23] [24]. Massive amounts of hepatic portal venous gas occurred due to the barotrauma during endoscopic procedure with balloon dilatation. The increased intra-luminal pressure causes mucosal tears within the bowel, which allows gas to enter submucosal veins and flow to the hepatic portal vein. A large amount of hepatic portal venous gas is not dangerous because the gas is not rapidly or easily transmitted to the central circulation. However, it may enter the inferior vena cava via the portal vein-hepatic artery capillary anastomosis when the gas is forced to be infused at a rapid and massive flow. The mechanical obstruction of the right ventricular pulmonary outflow tract and pulmonary vasculature occurs when a large amount of gas enters the right ventricle. Hepatic portal gas was observed in 20 cases with a 15% mortality rate, in the published case reports and our local case series. | ||||||
|
Conclusion
| ||||||
|
Gas embolus does produce clinical signs and symptoms which may mimic an acute cardiopulmonary or cerebrovascular event. The spectrum of vascular gas causes detected with MDCT is widening. Radiologists as well as emergency department physicians, should be familiar with the increasing number of vascular gas causes in addition to knowledge of the patient's clinical history. A careful search for clues on MDCT images was useful in discussing the etiology of vascular gas entry points and increased awareness of the emergent clinical settings where the vascular gas occurred. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Kazuya Sugimori – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Izumi Torimoto – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Kyota Nakamura – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Masaaki Kondo – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Kazushi Numata – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Shigeo Takebayashi – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2016 Kazuya Sugimori et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|